Ascyrus Medical Dissection Stent (AMDS) to treat acute Type A aortic dissections
Patients with acute type A aortic dissections present emergently to the hospital with a life-threatening problem of their aorta.
We have discussed this condition in detail here, here and here.
Virtually every patient with an acute Type A aortic dissection that survives the sudden onset of the problem and makes it to the hospital requires urgent surgical repair.
There are many surgical treatment options for repairing the aortic dissection. The choice of which procedure to perform in each patient is individualized based on the anatomy of the aortic dissection, the association of any medical complications of the aortic dissection and surgeon preference.
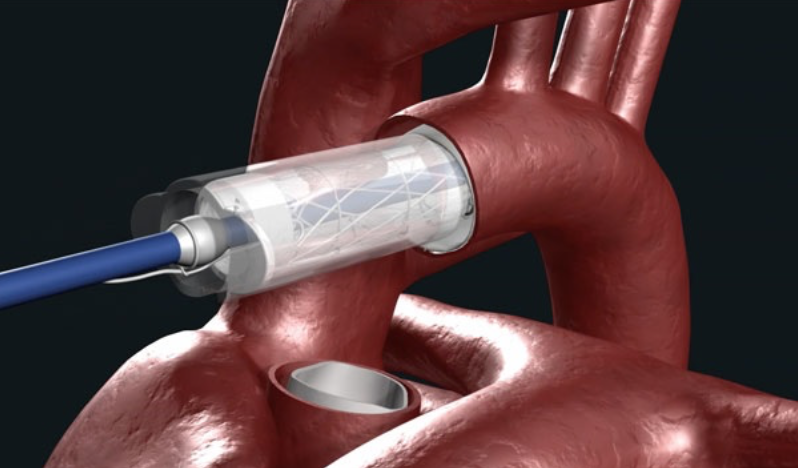
A new technology has been developed to expand the available treatment options for patients with acute type A aortic dissection called the Ascyrus Medical Dissection Stent (AMDS).
This innovative technology consists of 2 parts.
First, there is a a bare metal stent which is placed in the aortic arch and proximal descending thoracic aorta which physically pushes the dissection septum back against the aortic adventitia to allow the “healing” of the aortic dissection.
Second, there is a a felt cuff on the proximal end of the stent which facilitates the anastomosis of the proximal aortic repair to the stent located in the aortic arch.
The AMDS is to be used during open heart surgery procedures and is not a totally endovascular procedure.
You can watch a video of an animation of the procedure on here.
Several research publications have been published providing information about the clinical success achieved with this technology.
One published study is entitled “Dissected Aorta Repair Through Stent Implantation trial: Canadian results” in The Journal of Thoracic and Cardiovascular Surgery.
16 consecutive patients with acute type A aortic dissection were treated with the AMDS with a 30-day mortality of 6.3%. 91.7% of the patients and positive remodeling of the aortic arch and proximal descending thoracic aorta.
This designation is a very exciting development and will help accelerate continued study of this technology in the United States.
Stay Educated
Sign up for my weekly newsletter to stay educated about new developments in aortic disease.
Take a deep dive into learning more about aortic dissections and get your copy of my book BadAorta Volume One: A Deep Dive into Understanding Aortic Disease, It’s Treatments, and The Latest Research.

BadAorta Volume One: Aortic Dissections: A Deep Dive Into Aortic Disease, It’s Treatment And The Latest Research
Was this post informative?
Subscribe to my newsletter to learn more about the aorta, its diseases, and how to treat them.
Comments
Share your thoughts below — I try to get back to as many comments as possible.